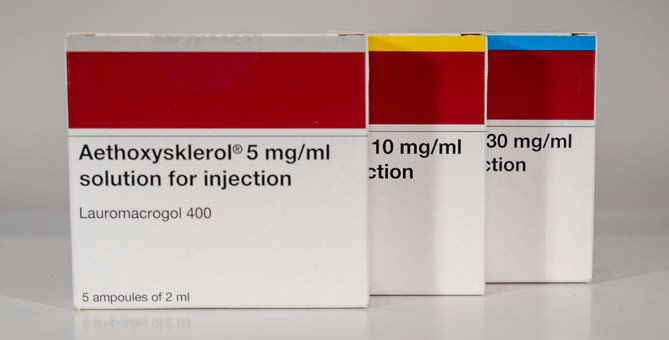
Aethoxysklerol, the prescription only medicine for sclerotherapy treatments of spider, reticular and varicose veins, is now available from Wigmore Medical. To assist practitioners, information leaflets are now available comparing Fibrovein and Aethoxysklerol concentration and volumes for the treatment of the different sized leg veins.
Aethoxysklerol contains Polidocanol (also known as Lauramacrogol 400) and is available as 5mg/ml (0.5%), 10mg/ml (1.0%) and 30mg/ml (3.0%) sterile solutions for injection (2ml ampoules). The product is recommended in the European guidelines for sclerotherapy and is registered for use in more than 30 countries worldwide. Manu-factured in Germany by Kreussler Pharma and licensed in the UK in partnership with Ferndale Pharmaceuticals.
Clinical evidence
Some of the Aethoxysklerol clinical evidence highlights are detailed below:
• Aethoxysklerol microsclerotherapy treating spider and reticular veins with the 0.5% and 1.0% strengths resulted in 95% highly effective clearance and 84% patient sat-isfaction (measures taken 26 weeks after treatment cessation)
• An extensive study conducted by 98 experienced phlebologists over a two year period, assessed Aethoxysklerol (0.5%, 1.0% and 3.0%) in treating spider veins, smaller venules and varicose veins. Participants were asked to compare it to their prior experience with sodium tetradecyl sulphate (STS). Aethoxysklerol was considered more effective by 85% and to have a lower frequency of complications by 90%
• Aethoxysklerol foam sclerotherapy (1.0%) treating small varicose veins and the great saphenous vein produced clinically effective and well-tolerated clearance
• and the 1.0% strength was shown to be equally effective to 0.5% STS in treating 3-6mm veins
• Aethoxysklerol foam sclerotherapy (3.0%) of the great saphenous vein produced elimination of reflux in 96% of patients, three weeks after a single treatment. The mean occlusion length of the vein was 38cm
Support
Wigmore Medical can provide support information leaflets on:
• The concentration and dosage comparison between Fibrovein and Aethoxysklerol for the spider, reticular and varicose veins.
• Aethoxysklerol dosage for treatment of different veins. For the treatment of spider veins a maximum dosage of 28ml of Aethoxysklerol 0.5% (5mg/ml) can be possible in a single treatment.
Copies of the Aethoxysklerol SmPC are available on request, and we can provide sclerotherapy patient information leaflets for use in your clinics. Contact us about how Aethoxysklerol could help you with your sclerotherapy treatments.
References
1. Aethoxysklerol Summary of Product Characteristics.
2. Rabe E, Breu FX, Cavezzi A et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology 2014; 29 (6):338-354.
3. Rabe E, Schliephake D, Otto J et al. Sclerotherapy of telangiectases and reticular veins: a double-blind, randomised, comparative clinical trial of polidocanol, sodium tetradecyl sulphate and isotonic saline (EASI study). Phlebology 2010; 25:124-131.
4. Conrad P, Malouf GM, Stacey MC et al. The Australian Polidocanol (Aethoxysklerol) Study. DermatolSurg 1995; 21:334-336.
5. Rao J, Wildemore JK, Goldman MP et al. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose veins and telangiectatic leg veins. Dermatol Surg 2005; 31:631-635.
6. Hamel-Desnos C, Ouvry P, Benigni JP et al. Comparison of 1% and 3% polidocanol foam in ultrasound guided sclerothera

 Added to basket
Added to basket

 Unapplied Changes
Unapplied Changes